Alphabetical

Charles's Law
[sujeto]
The relationship between a gas’s volume (V) and its temperature (T), which was first observed by Jacques Charles. Charles’s Law states that for a fixed amount of gas at a constant pressure, the gas’s volume increases linearly as its absolute temperature increases.
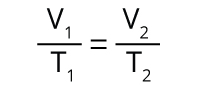
Ingresa o Registro
Para disfrutar de una experiencia sin publicidad y acceder a Visionlearning Classroom, inicie sesión o regístrese.
