Alphabetical

Boyle's Law
[noun]
The relationship between a gas’s volume (V) and pressure (P), which was first observed by Robert Boyle and Robert Hooke. Boyle’s Law states that for a fixed amount of gas at a stable temperature, the gas’s volume is inversely proportional to its pressure.
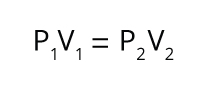
Appears in modules:
Sign in or register
For an ad-free experience and access the Visionlearning Classroom, sign in or register.
